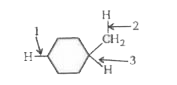
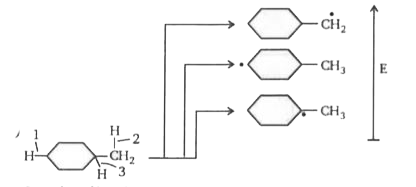
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Rank the bond dissociation energies of the bonds indicated with the ar...
Text Solution
|
- Rank the non-bonding electrons indicated by the arrows in order of inc...
Text Solution
|
- Explain each statement using resonance theory. (a) The indicated C-...
Text Solution
|
- Which has the largest bond dissociation energy?
Text Solution
|
- Which of the following bonds require the largest amount of bond energy...
Text Solution
|
- Assertion : Bond energy and bond dissociation energy have identical va...
Text Solution
|
- आबन्ध वियोजन ऊर्जा या बंध एन्थैल्पी किसे कहते है ?
Text Solution
|
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|
- In which of the following pairs, indicated bond having less bond disso...
Text Solution
|