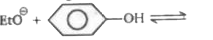
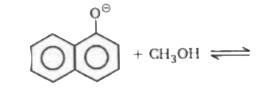
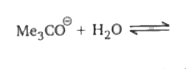A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the following acid-base reaction, in which can backward reaction if...
Text Solution
|
- K(p) for an endothermic chemical reaction is 10 atm. Then backward rea...
Text Solution
|
- In which of the following equilibrium system the rate of the backward ...
Text Solution
|
- In which of the following reactions, backward reaction I facoured ?
Text Solution
|
- Which of the following reactions favour backward direction ?
Text Solution
|
- In which of the following equlibrium system is the rate of the backwar...
Text Solution
|
- In which one of the following the increase of presure favours the back...
Text Solution
|
- Which of the following species can be formed in acid-base reactions as...
Text Solution
|
- In which of the following equilibrium system is the rate of the backwa...
Text Solution
|