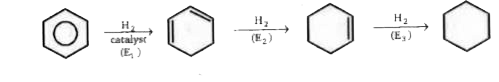
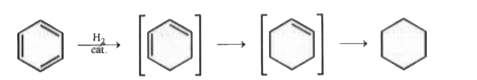
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- (E = activation energy) Relation between activation energies of abo...
Text Solution
|
- What is the relation between rate constant and activation energy of a ...
Text Solution
|
- State the role of activated complex in a reaction and state its relati...
Text Solution
|
- The activation energies for forward and backward reactions are Ef and ...
Text Solution
|
- Explain the relation between activation energy and the rate of the rea...
Text Solution
|
- State the role of activated complex in a reaction and state its relati...
Text Solution
|
- State the role of activated complex in a reaction and state its relati...
Text Solution
|
- State the role of activated complex in a reaction and state its relati...
Text Solution
|
- How is heat of reaction related to activation energy ?
Text Solution
|