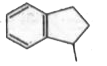
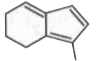
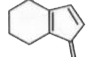
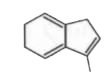
To determine which of the isomeric hydrocarbons is the most acidic, we need to analyze the structures of the given hydrocarbons and their ability to release a proton (H⁺).
### Step-by-Step Solution:
1. **Identify the Isomeric Hydrocarbons**:
- First, we need to look at the list of isomeric hydrocarbons provided in the question. For example, let’s assume we have three isomers: A, B, and C.
2. **Evaluate the Acidic Character**:
- The acidic character of a hydrocarbon is determined by its ability to lose a proton (H⁺). The more stable the resulting anion after the proton is lost, the more acidic the compound is.
3. **Consider Aromaticity**:
- Aromatic compounds are generally more stable due to resonance and delocalization of electrons. If losing an H⁺ leads to the formation of an aromatic compound, that compound will be more acidic.
- For instance, if one of the isomers is aromatic and the others are not, the aromatic compound will likely be the most acidic.
4. **Analyze Each Isomer**:
- For each isomer, consider what happens when you remove an H⁺:
- If removing H⁺ from isomer A leads to a non-aromatic structure, it is less acidic.
- If removing H⁺ from isomer B leads to an aromatic structure, it is more acidic.
- If removing H⁺ from isomer C leads to a non-aromatic structure, it is less acidic.
5. **Determine the Most Acidic Isomer**:
- After analyzing the stability of the anions formed from each isomer, we can conclude which isomer is the most acidic. In this case, if isomer B becomes aromatic upon losing H⁺, it will be the most acidic.
6. **Final Conclusion**:
- Based on the analysis, the isomer that becomes aromatic after the removal of H⁺ is the most acidic. Therefore, if isomer B is the one that becomes aromatic, it is the correct answer.
### Conclusion:
The most acidic isomeric hydrocarbon is the one that, upon losing a proton, becomes aromatic.