A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
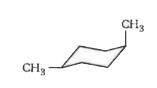
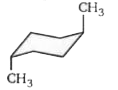
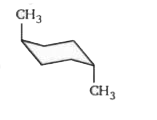
- The stable form of trans-1, 4 dimethylcyclohexane is represented as:
Text Solution
|
- The stable conformer (s) of trans -1-4- dimethyl] cyclohexane is/are:
Text Solution
|
- The most stable conformational isomer of trans-1-ethyl-2-methylcyclohe...
Text Solution
|
- Trans-1-2-dimethylcyclohexane
Text Solution
|
- Draw the most stable conformation of (a) 1,2-dimethylcyclohexane, (b) ...
Text Solution
|
- The most stable form of cis cyclohexane -1,3-diol is represented as :1
Text Solution
|
- Which would be the most stable conformation of trans-1-ethyl-3-methylc...
Text Solution
|
- Indigo shows cis-trans isomerism. Which is the stable form of Indigo
Text Solution
|
- Preface: Sis-3-chloropropio-2-nick acid is less stable in its trans fo...
Text Solution
|