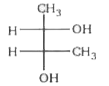
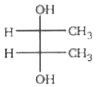
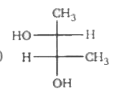
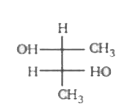
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following structure is not meso-2,3-butanediol ?
Text Solution
|
- Consider the following reactions : Meso-butan-2,3-diol is formed in :
Text Solution
|
- Which of the following structure (s) is/are meso 2,3 butanediol?
Text Solution
|
- a fischer projection of (2R,3S)-2,3-butanediol is
Text Solution
|
- Most stable from of meso-2,3-butandiol is :
Text Solution
|
- Among the following the Mnewmann projections of meso 2, 3 butanediol a...
Text Solution
|
- The meso form of 2,3 4- pentanetriol is :
Text Solution
|
- Which of the following structures repersent meso compound
Text Solution
|
- Identify which of the structures below are meso structures ?
Text Solution
|