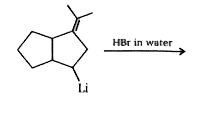
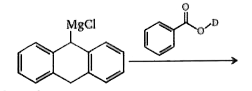
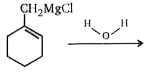A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In Which of the following reaction an acid-base reaction takes place ?
Text Solution
|
- Int the following acid-base reaction, in which can backward reaction i...
Text Solution
|
- The reactions of Krebs/citric acid cycle take place
Text Solution
|
- What happens when an acid reacts with a base? Explain by taking the ex...
Text Solution
|
- एक एल्कोहल और अम्ल के साथ होने वाली अभिक्रिया कहलाती ह...
Text Solution
|
- Which of the following reactions will not take place ?
Text Solution
|
- Which of the following reaction will not take place ?
Text Solution
|
- Which reaction is the reaction of an acid with a base?
Text Solution
|
- Which of the following reaction will not take place?
Text Solution
|