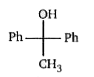
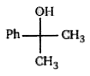
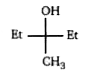
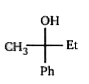
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following alcohol can not be prepared by the reaction of ...
Text Solution
|
- Which of the following alcohol can not be prepared by the reaction of ...
Text Solution
|
- Which of the following Grignard reagent can be prepared ?
Text Solution
|
- Why is Grignard reagent not successful in the preparation of ketones f...
Text Solution
|
- Assertion : Acid chlorides can not be converted to ketones by reacting...
Text Solution
|
- Which of the following alcohol (s) can be prepared by the action of Gr...
Text Solution
|
- Assertion: Both grignard reagent and dialkyl cadmium react with acid c...
Text Solution
|
- Which one of the alcohol cannot be prepared by grignard reagent?
Text Solution
|
- Ethyl alcohol can be prepared from grignard reagent by the reaction of...
Text Solution
|