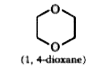
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compound is not a suitable solvent for Grignard...
Text Solution
|
- Which of the following compounds will form hydrocarbon on reaction wit...
Text Solution
|
- Which of the following compounds is not suitable solvent for Grignard ...
Text Solution
|
- Which of the following solvent is suitable for S(N)1 reaction ?
Text Solution
|
- Grignard reagent is most suitable for preparation of which of the foll...
Text Solution
|
- Ether is a good solvent for Grignard reagent. Which property makes it ...
Text Solution
|
- Which is used as solvent during reactions with Grignard reagent ?
Text Solution
|
- The compound that acts as a solvent for Grignard reagent is:
Text Solution
|
- Which solvent will be suitable for SN2 reaction?
Text Solution
|