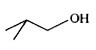
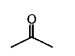
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- underset("(excess)")(CH(3)MgBr) + Et - O-overset(O)overset(||)(C)-O -E...
Text Solution
|
- CH(3)-overset(overset(O)(||))(C)-C Cl(3)underset("(excess)")overset(CH...
Text Solution
|
- Number of organic products formed in following feaction. H-underset(O)...
Text Solution
|
- Et-O-overset(O)overset(||)(C)-O-Etunderset((2)H(3)O^(o+))overset((1)CH...
Text Solution
|
- CH(3)-(CH(2))(3)-OH overset(CH(3)-underset(O)underset(||)overset(O)ove...
Text Solution
|
- H-overset(O)overset(||)C-overset(O)overset(||)C-Hoverset(CH(3)MgBr(exc...
Text Solution
|
- Et-O-overset(O)overset(||)C-CH(2)-CH(2)-overset(O)overset(||)C-O-Et ov...
Text Solution
|
- In the reaction sequence CH(3)-overset(O)overset(||)(C)-H-underset(.^(...
Text Solution
|
- In the following reaction sequence, CH(3) - underset((X))underset(OH...
Text Solution
|