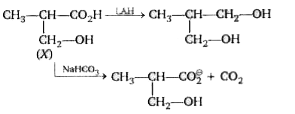
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An optically active compound 'X' has molecular formula C(4)H(8)O(3). I...
Text Solution
|
- Compound (A) (C(4)H(8)O(3)) reacts with NaHCO(3) and evolves CO(2)(g)....
Text Solution
|
- A compound (X) with the molecular formula C(3)H(8)O can be oxidized to...
Text Solution
|
- An optically active compound 'X' having molecular formula C(4)H(8)O(3)...
Text Solution
|
- A compound with molecular formula C(4)H(10)O(4) on acylation with acet...
Text Solution
|
- A compound X with molecular formula C(3)H(8)O can be oxidised to a com...
Text Solution
|
- Give the structures of the four optically active structural isoamers o...
Text Solution
|
- An optically active compound 'X' evolve CO(2) with NaHCO(3). The 'X' ...
Text Solution
|
- What is the number of optically active structural isomers of C(4)H...
Text Solution
|