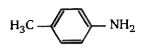
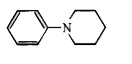
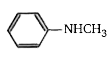
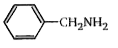A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which amine yields N-nitroso amine after treatment with nitrous acid (...
Text Solution
|
- Which of the following amines form N- nitroso derivative when treated ...
Text Solution
|
- Treatment of phenol with nitrous acid (NaNO(2)+HCl) gives mainly
Text Solution
|
- The amine which will not liberate nitrogen on reaction with nitrous ac...
Text Solution
|
- Primary amine on treatment with NaNO(2) and HCl yields :
Text Solution
|
- एथिल ऐमीन पर नाइट्रस अम्ल अथवा NaNO(2)//HCl की क्रिया से प्राप्त होने ...
Text Solution
|
- यौगिक जो निम्न ताप पर जलीय नाइट्रस अम्ल के साथ अभिक्रिया करके तेलीय ना...
Text Solution
|
- द्वितीयक ऐमीनस नाइट्रस अम्ल से किस प्रकार क्रिया हारते है? उदाहरणों को...
Text Solution
|
- Diethyl amine when treated with nitrous acid yields
Text Solution
|