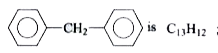
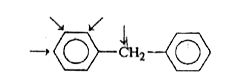A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The molecular formula of diphenylmethane, How many structural iso...
Text Solution
|
- The molecule formula of diphenyl methane is C(13)H(12) . How many stru...
Text Solution
|
- The molecular formula of diphenylmethane, How many structural isomers ...
Text Solution
|
- The molecular formula of diphenyl methane, is C(13)H(12). How many ...
Text Solution
|
- How many stuctural isomers are possible when one of the hydrogen is re...
Text Solution
|
- The formula of disheylnethane How many structural are possible w...
Text Solution
|
- How many structural isomers are possible when one of the hydrogen in c...
Text Solution
|
- How many structural isomers are possible when one of the hydrogen is r...
Text Solution
|
- The molecular formula of dipheylmethane is C(13)H(12) How many structu...
Text Solution
|