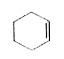
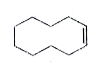
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Geometrical isomerism is possible in :
Text Solution
|
- Geometrical isomerism is not possible in
Text Solution
|
- In co-ordination complex geometrical isomerism is possible for complex
Text Solution
|
- In which of the following geometrical isomerism is possible ?
Text Solution
|
- Isomerism - Geometrical Isomerism
Text Solution
|
- Isomerism - Geometrical Isomerism 2
Text Solution
|
- Explain about the geometrical isomerism possible in oximes.
Text Solution
|
- In which of the following geometrical isomerism is possible ?
Text Solution
|
- Geometrical isomerism may be possible with
Text Solution
|