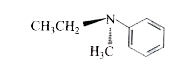To determine which of the given compounds is optically inactive, we need to analyze each compound for the presence of a plane of symmetry and consider the concept of optical activity.
### Step-by-Step Solution:
1. **Understanding Optical Activity**:
- A compound is optically active if it can rotate plane-polarized light. For a compound to be optically active, it must lack a plane of symmetry and possess chiral centers.
2. **Analyzing the First Compound (Ethyl, Methyl, Phenyl Amine)**:
- The structure includes ethyl (C2H5), methyl (CH3), and phenyl (C6H5) groups attached to a nitrogen atom.
- The nitrogen has a lone pair of electrons.
- There is no plane of symmetry in this compound, making it potentially optically active.
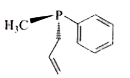
3. **Analyzing the Second Compound (Allyl Group with Phenyl)**:
- The structure features a methyl group (CH3) in a wedge position, an allyl group in a dash position, and a phenyl group in the planar position.
- The presence of the lone pair on nitrogen again means there is no plane of symmetry, suggesting it could be optically active.
4. **Analyzing the Third Compound (Allene)**:
- The structure is an allene with groups pH (phenyl), CH3, Cl, and Br.
- In allenes, the two double bonds create perpendicular planes for the substituents, which means there is no plane of symmetry. Thus, this compound is also potentially optically active.
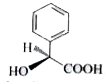
5. **Analyzing the Fourth Compound (Phenyl Carboxylic Acid)**:
- The structure includes a phenyl group attached to a carboxylic acid (COOH) with a hydrogen and hydroxyl (OH) group.
- In this case, the compound can exhibit amine inversion due to the nitrogen's lone pair, leading to the formation of a racemic mixture of enantiomers (equal amounts of two enantiomers).
- Since both enantiomers are present in equal amounts, the compound is optically inactive.
6. **Conclusion**:
- After analyzing all four compounds, we find that the first compound (ethyl, methyl, phenyl amine) undergoes amine inversion, leading to optical inactivity due to the presence of equal amounts of enantiomers. Therefore, the optically inactive compound is **option 1**.
### Final Answer:
The optically inactive compound is **option 1: Ethyl, Methyl, Phenyl Amine**.