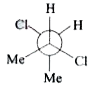
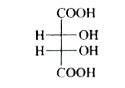A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following will not show optical isomerism ?
Text Solution
|
- Which of the following will show optical isomerism? .
Text Solution
|
- Which of the following will show optical isomerism?
Text Solution
|
- Which one of the following will show optical isomerism?
Text Solution
|
- Which of the following will show optical isomerism?
Text Solution
|
- निम्न में से कौन सा संकुल प्रकाशिय समावयवता नहीं दर्शाता है
Text Solution
|
- निम्न में से कोन-सा संकुल प्रकाशिक समावयावता प्रदर्शित करेगा?
Text Solution
|
- Which of the following shows optical isomerism ?
Text Solution
|
- Which of the following will show optical isomerism?
Text Solution
|