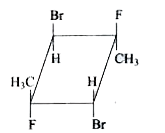
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compound has :
Text Solution
|
- The compound 1,2- butadiene has :
Text Solution
|
- The compound 1,3-butadiene has
Text Solution
|
- An organic compound has the structure It will give
Text Solution
|
- Explain why compound A has no enantiomer and why compound B has no dia...
Text Solution
|
- Compound (II) is/has
Text Solution
|
- The given compound (X) has:
Text Solution
|
- An organic compound has the structure
Text Solution
|
- The compound 1,2-butadiene has :
Text Solution
|