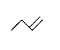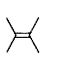A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Cis-trans isomerism is shown by:
Text Solution
|
- Which of the following not show cis-trans isomerism?
Text Solution
|
- Which of the following cycloalkanes will shown cis-trans isomerism?
Text Solution
|
- Cis-trans isomerism is not possible in alkynes because of :-
Text Solution
|
- Which can show the cis-trans isomerism:
Text Solution
|
- In cis- trans isomerism, the compound generally
Text Solution
|
- Amongst the following cis - trans isomerism in shown by
Text Solution
|
- But-2-ene exhibits cis-trans-isomerism due to
Text Solution
|
- Cis-trans isomerism is exhibited by:
Text Solution
|