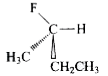
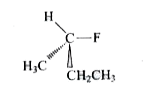
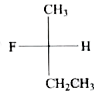
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The structure of (S)-2-fluorobutane is best represented by:
Text Solution
|
- The structure of aluminium bromide is best represented as
Text Solution
|
- The most stable conformation of 3-fluorobutan-2-ol-is
Text Solution
|
- Explain why CO(3)^(2-) ion cannot be represented by a single Lewis str...
Text Solution
|
- Explain why CO(3)^(2-) ion cannot be represented by a single Lewis str...
Text Solution
|
- The structure of ozone can best be represented by
Text Solution
|
- Explain why CO(3)^(2-) ion cannot be represented by a single Lewis str...
Text Solution
|
- The cell reaction Zn(s) + Cu^(+2)rarr Zn^(+2) + Cu(s) is best represen...
Text Solution
|
- The structure of Fe(2)Cl(6) is best represented as :
Text Solution
|