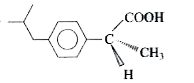
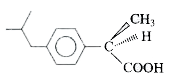
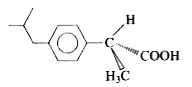
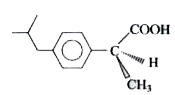
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The S enantiomer of ibuprofen is responsible for its pain-relieving pr...
Text Solution
|
- The analgesic drug ibuprofen (A) is chiral and exists in (+) and (-) f...
Text Solution
|
- The analgesic drug ibuprofen (A) is chiral and exists in (+) and (-) f...
Text Solution
|
- The analgesic drug ibuprofen (A) is chiral and exists in (+) and (-) f...
Text Solution
|
- The analgesic drug ibuprofen (A) is chiral and exists in (+) and (-) f...
Text Solution
|
- Ibuprofen is:
Text Solution
|
- The S-enantiomers of ibuprofen is reponsible for its pain-relieving pr...
Text Solution
|
- Ibuprofen is a
Text Solution
|
- The S-ibuprofen is responsible for its pain relieving property. Which ...
Text Solution
|