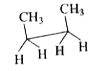
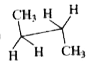
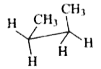
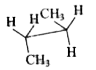
To determine which conformation of n-butane has a center of symmetry, we will analyze each option provided. A center of symmetry is defined as a point in a molecule where for every atom or group of atoms, there is an equivalent atom or group at an equal distance on the opposite side.
### Step-by-step Solution:
1. **Understand the Definition of Center of Symmetry**:
- A center of symmetry is a point in a molecule where all groups reflect each other in a straight line. For a molecule to have a center of symmetry, for every atom or group on one side of the center, there must be an identical atom or group on the opposite side.
2. **Analyze Option A**:
- The conformation in option A shows a sawhorse projection with groups arranged as follows: CH3, CH3, H, H, H, H.
- Upon inspection, it is evident that there is no point where all groups can be reflected across a center. Therefore, **Option A does not have a center of symmetry**.
3. **Analyze Option B**:
- The structure in option B has the following arrangement: CH3, H, H on one carbon and CH3, H, H on the other carbon.
- If we consider a point in the middle of the two carbons, we can see that:
- The CH3 group on one side reflects to the CH3 group on the opposite side.
- The H atoms also reflect across this point.
- Therefore, **Option B has a center of symmetry**.
4. **Analyze Option C**:
- The conformation in option C has the arrangement: CH3, CH3, H, H.
- Here, there is no point where all groups can be symmetrically reflected across a center. Thus, **Option C does not have a center of symmetry**.
5. **Analyze Option D**:
- The structure in option D shows: H, H, CH3 on one side and CH3, H, H on the other.
- Similar to option C, there is no point where all groups can reflect each other symmetrically. Hence, **Option D does not have a center of symmetry**.
6. **Conclusion**:
- After analyzing all options, we find that the only conformation of n-butane that has a center of symmetry is **Option B**.
### Final Answer:
The correct answer is **Option B**.