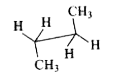
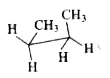
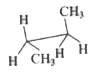
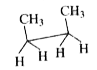A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following conformations of n-butane has a plane of symmet...
Text Solution
|
- Which of the following conformer of butane has minimum energy?
Text Solution
|
- In the following the most stable conformation of n-butane is:
Text Solution
|
- Which conformation of n-Butane has both plane of summetry and centre o...
Text Solution
|
- Which conformer of n-butane is chiral?
Text Solution
|
- which of the following conformations of -n- butane is least stabel ?
Text Solution
|
- Which of the following conformers of n-butane has torsional strain?
Text Solution
|
- In the following the most stable conformation of n-butane is :
Text Solution
|
- In the following the most stable conformation m-butane is:
Text Solution
|