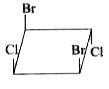
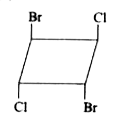
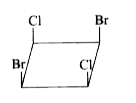
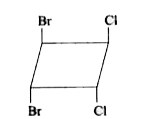
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following structures is chiral?
Text Solution
|
- Which of the following has chiral structure?
Text Solution
|
- Which of the following structures has the R-configuration at the chir...
Text Solution
|
- Which of the following structures has the S-configuration at the chira...
Text Solution
|
- Which of the following structures represents a chiral compound?
Text Solution
|
- Which of the structures given below are chiral ?
Text Solution
|
- Examine the following structural formulas and select those that are ch...
Text Solution
|
- Which of the following isomer of butanol has a chiral structure?
Text Solution
|
- Which of the following structures does not contain any chiral C atom b...
Text Solution
|