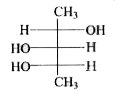
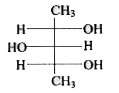
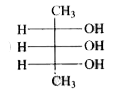
To determine the meso form of 2,3,4-pentanetriol, we need to follow these steps:
### Step 1: Understand the Structure of 2,3,4-Pentanetriol
2,3,4-pentanetriol is a five-carbon chain (pentane) with hydroxyl (OH) groups attached to the second, third, and fourth carbon atoms. The structure can be represented as follows:
```
CH3-CH(OH)-CH(OH)-CH(OH)-CH3
```
### Step 2: Identify Chiral Centers
A chiral center is a carbon atom that is attached to four different groups. In 2,3,4-pentanetriol, the second, third, and fourth carbons are all chiral centers because they each have an OH group, a hydrogen atom, and two different carbon chains attached.
### Step 3: Determine the Meso Form
A meso compound is a molecule that has multiple chiral centers but is superimposable on its mirror image due to an internal plane of symmetry. To find the meso form, we need to visualize the molecule and look for a plane of symmetry.
1. **Draw the molecule in 3D**: Visualize or sketch the molecule in a way that shows the spatial arrangement of the groups around the chiral centers.
2. **Identify the plane of symmetry**: If you can draw a line through the molecule that divides it into two mirror-image halves, then the molecule is meso.
### Step 4: Analyze Possible Structures
1. **Structure A**: Check if there is a plane of symmetry. If the OH groups are not positioned symmetrically, this structure is not the meso form.
2. **Structure B**: Check for symmetry. If the OH groups and other substituents are arranged symmetrically, this could be the meso form.
3. **Structure C**: Similar analysis as above.
### Step 5: Conclusion
After analyzing the structures, the meso form of 2,3,4-pentanetriol is the one that has a plane of symmetry and is optically inactive due to the presence of this symmetry.
### Final Answer
The meso form of 2,3,4-pentanetriol is the structure that has both a plane of symmetry and is superimposable on its mirror image.
---