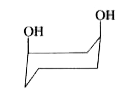
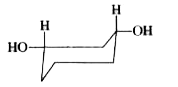
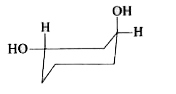
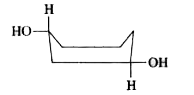
To determine which of the given conformations is the most stable, we need to analyze the interactions present in each conformation. Let's break down the analysis step by step.
### Step-by-Step Solution:
1. **Identify the Conformations**:
- We have four conformations labeled A, B, C, and D. Each conformation has different orientations for the hydroxyl (OH) groups.
2. **Analyze Conformation A**:
- In conformation A, both OH groups are in the axial position.
- When we draw the hydrogen atoms in this conformation, we notice that there is a hydrogen bonding interaction between one of the axial hydrogens and the oxygen of the OH group.
- This interaction is stabilizing, making conformation A potentially stable.
3. **Analyze Conformation B**:
- In conformation B, both OH groups are in the equatorial position.
- There are no significant interactions between the OH groups in this conformation, which means it lacks stabilizing or destabilizing interactions.
4. **Analyze Conformation C**:
- In conformation C, one OH group is in the axial position and the other is in the equatorial position.
- The axial OH group experiences 1,3-diaxial repulsion with the axial hydrogens, leading to destabilizing interactions. This makes conformation C less stable.
5. **Analyze Conformation D**:
- In conformation D, one OH group is axial and the other is equatorial, similar to conformation C.
- However, there are no significant interactions present in this conformation either, which means it also lacks stabilizing interactions.
6. **Compare Stability**:
- Conformation A has stabilizing hydrogen bonding interactions.
- Conformations B and D have no interactions, while conformation C has destabilizing interactions.
- Therefore, conformation A is the most stable due to the presence of hydrogen bonding.
### Conclusion:
The most stable conformation among the given options is **Conformation A**, which has hydrogen bonding interactions.
---