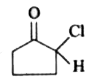
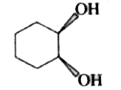
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following molecules is expected to rotate the plane polar...
Text Solution
|
- Which of the following molecules is expected to rotate the plane polar...
Text Solution
|
- Which of the following compound will rotate the plane polarized light ...
Text Solution
|
- Which one of the following is expected to rotate the plane of plane po...
Text Solution
|
- Which of the following molecule is expected to rotate plane polarised ...
Text Solution
|
- Rotation of plane polarized light is measured by
Text Solution
|
- Which of the following molecules is expected to rotate the plane of po...
Text Solution
|
- निम्नलिखित में से कौन-सा यौगिक समतल ध्रुवित प्रकाश को घुमा नहीं सकता?
Text Solution
|
- Which one of the following rotates the plane polarized light towards l...
Text Solution
|