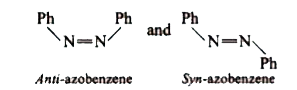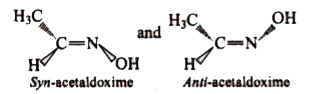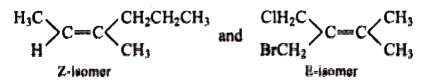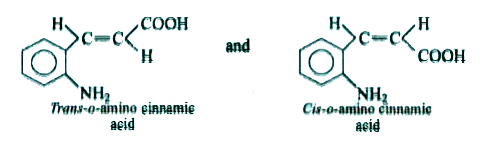A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For which of the following pairs of compounds are the correct notation...
Text Solution
|
- The correct relationship among the following pairs of given compound i...
Text Solution
|
- Which of the following is correct for the given compound
Text Solution
|
- Which of the following statement(s) is/are correct for given compound ...
Text Solution
|
- Consider the following pair of compound which of the followin...
Text Solution
|
- Which of the following represents the correct of the acidity in the gi...
Text Solution
|
- Select the correct pair from the following given pair.
Text Solution
|
- On the reaction of an alkyne with a reagent, the above given compound ...
Text Solution
|
- Which of the following are correct notations for the orbitals ?
Text Solution
|