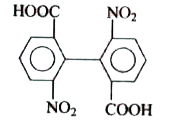
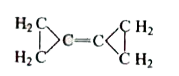
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds exhibit optical isomerism?
Text Solution
|
- Which of the following compounds will exhibit optical isomerism ?
Text Solution
|
- Among the following compounds which exhibits optical isomerism
Text Solution
|
- Which of the following compounds can exhibit optical isomerism ?
Text Solution
|
- Which of the following compounds will exhibit optical isomerism ?
Text Solution
|
- निम्न में से कौन-सा यौगिक प्रकाशिक समावयवता प्रदर्शित कर सकता है ?
Text Solution
|
- कौन-सा संकर यौगिक प्रकाशिक समावयवता प्रदर्शित करता है ?
Text Solution
|
- निम्नलिखित में से कौन-सा यौगिक प्रकाशिक समावयवता प्रदर्शित नहीं करता ह...
Text Solution
|
- निम्नलिखित में से कौन-सा यौगिक प्रकाशिक समावयवता प्रदर्शित नहीं करता ह...
Text Solution
|