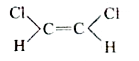
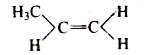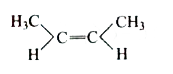A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following will have a trans isomer?
Text Solution
|
- If (A) and (C) show geometrical isomers but not optical isomers, and (...
Text Solution
|
- Determine whether each of the followings compounds is a cis isomer or ...
Text Solution
|
- Determine whether each of the followings compounds is a cis isomer or ...
Text Solution
|
- Which of the following alkene on catalytic hydrogenation gives cis and...
Text Solution
|
- Which of the following aquare planar complex ions can have cis-trans i...
Text Solution
|
- How many statements are true for the following pair of compounds ? ...
Text Solution
|
- Write true or false. Cis-trans isomers have different dipole moments
Text Solution
|
- Which isomers among cis and trans have highest boiling point?
Text Solution
|