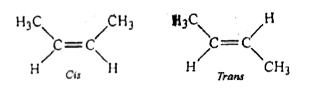A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Cis-2-butene and trans-2-butene are.
Text Solution
|
- Among the following alkenes: {:(1-"Butene,",cis-2-"Butene",,"trans"-2-...
Text Solution
|
- Assertion:- Boiling point of cis-2-butene is more than trans-2-butene....
Text Solution
|
- What characterstic is the best common to both cis-2-butene and trans-2...
Text Solution
|
- If a mixture containing 1-butene, cis-2-butene and trans-2-butene is t...
Text Solution
|
- Which has the least dipole moment-1 -butene, cis-2-butene, trans-2-but...
Text Solution
|
- The compounds cis - 2 - butene and trans - 2 - butene can be different...
Text Solution
|
- Correct order of stability is (A) Cis-2-butene > 1 - butene > trans - ...
Text Solution
|
- (A) cis-2-butene overset(HCO3H)rarrI (B) trans-2-butene overset(HCO3H)...
Text Solution
|