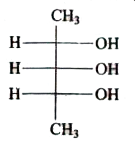
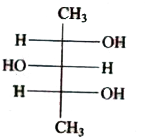
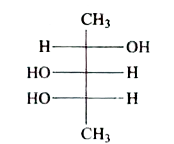
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds can have superimposable mirror image?
Text Solution
|
- Isomers which are …… mirror images are knows as ….. (superimposable, n...
Text Solution
|
- Which of the following compound has non superimposable mirror image -
Text Solution
|
- Which of the following compounds can non-superimposable mirror images?
Text Solution
|
- Which of the following molecule has non-superimposible mirror images ?
Text Solution
|
- Molecules whose mirror image is non-superimposable over them are known...
Text Solution
|
- If mirror image of the compouns is not superimposable on it, most appr...
Text Solution
|
- Stereoisomers which are not mirror image of each other, are called.:
Text Solution
|
- Which of the following is non - superimposable on its mirror image-
Text Solution
|