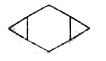
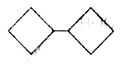
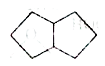A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the compounds shown below are isomers?
Text Solution
|
- Which compounds shown below has six isomers?
Text Solution
|
- From the compound shown below, choose which is/are aromatic.
Text Solution
|
- Which combination is best for preparation of the compound (A) shown be...
Text Solution
|
- Which of the following is the enantiomer of the compound shown below ?
Text Solution
|
- The two compounds shown below are :
Text Solution
|
- The compounds shown below are
Text Solution
|
- " Number of geometrical isomers for the below compound "
Text Solution
|
- Which pair of isomers given below are position isomers?
Text Solution
|