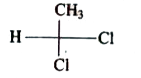
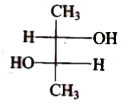
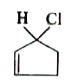
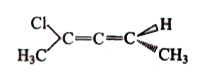A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Presence of chiral center is not an essential condition to show optica...
Text Solution
|
- Presence of chiral center is not an essential condition to show optica...
Text Solution
|
- प्रकाशिक समावयवता दर्शाने वाला यौगिक है :
Text Solution
|
- प्रकाशिक समावयवता दर्शाने वाला यौगिक है :
Text Solution
|
- प्रकाशिक समावयवता दर्शाने वाला यौगिक है :
Text Solution
|
- An organic compound will show optical isomerism if .
Text Solution
|
- Which compounds will show optical isomerism
Text Solution
|
- The essential condition for a molecule to exhibit optical isomerism is...
Text Solution
|
- निम्न में से कौनसा उपसहसंयोजी यौगिक प्रकाशीय समावयवता दर्शायेगा
Text Solution
|