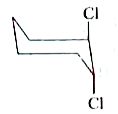
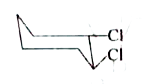
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Cyclohexane exists its two chair conformations in rapid equilrium at r...
Text Solution
|
- Assertion : Cis-1,3 dihydroxy cyclohexane exists in chair conformation...
Text Solution
|
- Cyclohexane exist as two chair conformations in rapid equilibrium at r...
Text Solution
|
- Cyclohexane exist as two chair conformations in rapid equilibrium at r...
Text Solution
|
- Stability of cycloalkanes can be explained on the basis of Bayer stain...
Text Solution
|
- Stability of cycloalkanes can be explained on the basis of Bayer stain...
Text Solution
|
- Assertion : Cis-1,3 dihydroxy cyclohexane exists in chair conformation...
Text Solution
|
- Assertion : In trigonal bipyramidal structure two axial bonds are long...
Text Solution
|
- The number of axial hydrogen atoms in chair form of cyclohexane is
Text Solution
|