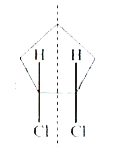
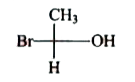
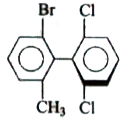
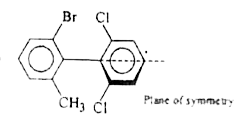
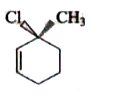A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A line which bisects a compound in two equal parts and both parts humo...
Text Solution
|
- Which of the following compounds does not contain the plane of symmetr...
Text Solution
|
- A line which bisects a compound in two equal parts and both parts appe...
Text Solution
|
- A line which bisects a compound in two equal parts and both parts appe...
Text Solution
|
- A line which bisects a compound in two equal parts and both parts appe...
Text Solution
|
- In which of the following compound, prossess plane os symmetry as well...
Text Solution
|
- Which of the following molecules has axis of symmetry and a coaxial pl...
Text Solution
|
- Which of the following has plane of symmetry:-
Text Solution
|
- If a molecule contains one carbon atom carrying four different groups ...
Text Solution
|