A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
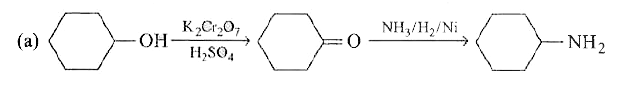
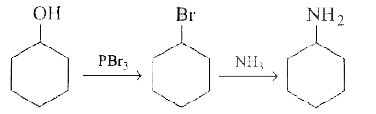
- Cyclohexanol can be converted into cyclohexylamine by following two ro...
Text Solution
|
- Which of the following reaction is expected to give readily a hydrocar...
Text Solution
|
- Which of the following reaction is expected to readily gives hydrocarb...
Text Solution
|
- Which of the following reactions is expected to radily give a hydrocar...
Text Solution
|
- Which of the following reactions would give a good yield of hydrocarbo...
Text Solution
|
- Action of NaNO(2)+ dil HCl on ArNH(2) yields ArN(2)^(+)Cl^(-) . A simi...
Text Solution
|
- Suggest chemical reactions for the following conversions: (i) Cycloh...
Text Solution
|
- Which of the followeing reactions is expected to readily give a hyroca...
Text Solution
|
- Give reason: Aniline is a weaker base than cyclohexylamine.
Text Solution
|