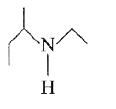
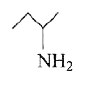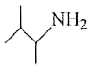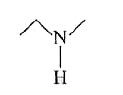A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of following compounds exists as non-resolvable racemic mixture?
Text Solution
|
- Which of the following is a racemic mixture?
Text Solution
|
- Which of the following compounds exsits as a nonresolvable racemic mix...
Text Solution
|
- The most important chemical method of resolve a racemic mixture makes ...
Text Solution
|
- Which of the following compounds exists a nonresolvable racemic mixtur...
Text Solution
|
- Racemic modification can be resolved by
Text Solution
|
- Which of the following compounds will give racemic mixture by S(N^(1))...
Text Solution
|
- Which of the following reaction will give racemic mixture ?
Text Solution
|
- Which of the following compounds will give racemic mixture on nucleoph...
Text Solution
|