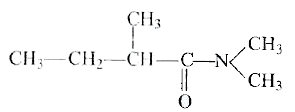
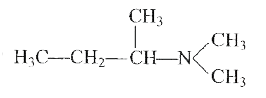A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- H(3) C - CH(2) - overset(overset(CH3) (|))CH - underset(underset(O)(||...
Text Solution
|
- H(3)C-CH(2)-overset(CH(3))overset(|)(CH)-underset(O)underset(||)(C)-NH...
Text Solution
|
- CH(3)-overset(O)overset(||)(C)-CH(2)-CH(2)-overset(O)overset(||)(C)-CH...
Text Solution
|
- CH(3)-overset(O)overset(||)(C)-OHoverset(ND(3))underset(Delta)(to)(A)o...
Text Solution
|
- CH(3) overset(CH(3))overset(|)underset(CH(3))underset(|)(C) -Br overse...
Text Solution
|
- Identify the compound 'D' in the following series of reactions . CH(3)...
Text Solution
|
- Identify the compound 'D' in the following series of reactions . CH(...
Text Solution
|
- CH(3)-underset(CH3)underset(|)overset(CH3)overset(|)(C )-underset(Br)u...
Text Solution
|
- CH3-overset(CH3)overset(|)underset(OH)underset(|)C-overset(CH3)overset...
Text Solution
|