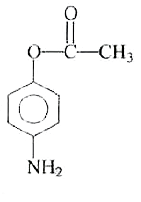
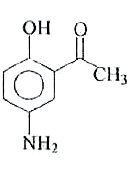
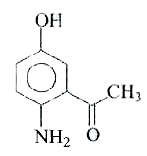
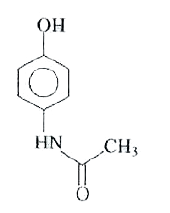
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The reaction of p-aminophenol with one mole of acetyl chloride in pres...
Text Solution
|
- The reaction of p- aminophenol with one equivalent of acety1 chloride ...
Text Solution
|
- Reaction of R-2- butanol with p-toluenesulphonyl chloride in pyridine ...
Text Solution
|
- The reaction of aromatic acyl chloride and phenol in the presence of a...
Text Solution
|
- Aniline on reaction with acetyl chloride gives
Text Solution
|
- रासायनिक रूप से पैरासीटामोल -ऐसीटिल- -ऐमीनोफीनॉल है ।
Text Solution
|
- Drug prepared by acetylation of p-aminophenol which is both an analges...
Text Solution
|
- Aniline on reaction with acetyl chloride gives
Text Solution
|
- Aniline reacts with acetyl chloride in presence of pyridine giving―
Text Solution
|