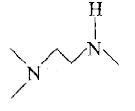
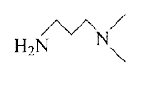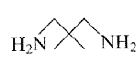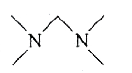A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The Hinsberg's test of a compound, C5H(14)N2 produces a solid that is ...
Text Solution
|
- Length of a rectangular solid is increased by 10% and breadth is de...
Text Solution
|
- A compound with formula C5H(13) gives a base soluble derivative iin th...
Text Solution
|
- A nitrogen containg compound dissolves is 10% aqueous sulphuric acid. ...
Text Solution
|
- The Hinsberg test of a C(5)H(14)N(2) compound produces a solid that i...
Text Solution
|
- Which of the following compounds will not dissolve in aqueous NaOH?
Text Solution
|
- Which of the following compounds will dissolve in aq. NaOH ?
Text Solution
|
- In which one of the following ways would the pH value of aqueous ammon...
Text Solution
|
- किसी यौगिक C(5)H(14)N(2) के हिन्सबर्ग परीक्षण पर एक ऐसा ठोस उतपन्न ह...
Text Solution
|