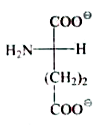
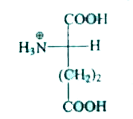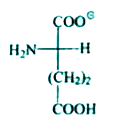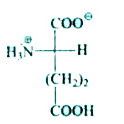A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is the major solute species in a solution of gl...
Text Solution
|
- In decinormal solution, CH(3)COOH acid is ionised to the extent of 1.3...
Text Solution
|
- Which of the following is the major solute species in a solution of al...
Text Solution
|
- Which of the following is the major solute species in a solution of ly...
Text Solution
|
- The pH of the solution containing following zwitter ion species is
Text Solution
|
- The pH of the solution containing following zwitter ion species is
Text Solution
|
- Assume that a particular amino acid has an isoelectric point of 6.0. I...
Text Solution
|
- Which is more acidic: a solution of pH=2 or a solution of pH=6?
Text Solution
|
- Which is a stronger acid ? A solution with pH 5 and a solution with pH...
Text Solution
|