A white crystalline solid `X` give following chemical test :
(i) it liberates `CO_(2)` with `NaHCO_(3)`
(ii) it forms a coloured dye on diazotisation and coupling with `beta` - naphthol
(iii) with `Br_(2)` water it forms white precipitate of `2,4,6` - tribromo aniline .
`'X'` can be identified as :
A white crystalline solid `X` give following chemical test :
(i) it liberates `CO_(2)` with `NaHCO_(3)`
(ii) it forms a coloured dye on diazotisation and coupling with `beta` - naphthol
(iii) with `Br_(2)` water it forms white precipitate of `2,4,6` - tribromo aniline .
`'X'` can be identified as :
(i) it liberates `CO_(2)` with `NaHCO_(3)`
(ii) it forms a coloured dye on diazotisation and coupling with `beta` - naphthol
(iii) with `Br_(2)` water it forms white precipitate of `2,4,6` - tribromo aniline .
`'X'` can be identified as :
A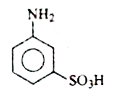

B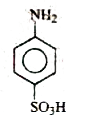

C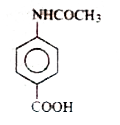
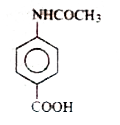
D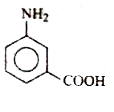

Text Solution
AI Generated Solution
The correct Answer is:
To identify the white crystalline solid `X`, we will analyze the given chemical tests step by step.
### Step 1: Analyzing the first test
The first test states that `X` liberates `CO₂` with `NaHCO₃`. This reaction indicates that `X` must contain a carboxylic acid group (`-COOH`) or a sulfonic acid group (`-SO₃H`). Both of these functional groups can react with sodium bicarbonate to release carbon dioxide.
**Hint**: Look for compounds that contain either a carboxylic acid or a sulfonic acid group.
### Step 2: Analyzing the second test
The second test indicates that `X` forms a colored dye upon diazotization and coupling with `β-naphthol`. This suggests that `X` must contain an amino group (`-NH₂`), as diazotization is a characteristic reaction of aniline derivatives. The reaction involves converting the amino group into a diazonium salt, which can then couple with `β-naphthol` to form a colored compound.
**Hint**: Identify compounds that have an amino group (`-NH₂`) attached to a benzene ring.
### Step 3: Analyzing the third test
The third test states that `X` reacts with `Br₂` water to form a white precipitate of `2,4,6-tribromoaniline`. This reaction is characteristic of aniline derivatives, where the amino group is highly reactive towards electrophilic substitution. The presence of the amino group allows for bromination at the ortho and para positions, leading to the formation of `2,4,6-tribromoaniline`.
**Hint**: Confirm that the compound can undergo bromination at the ortho and para positions due to the presence of the amino group.
### Step 4: Evaluating the options
Now, we can evaluate the options based on the information gathered:
- **Option A**: Contains a `-COOH` group but lacks an `-NH₂` group.
- **Option B**: Contains both `-COOH` and `-NH₂` groups, making it a suitable candidate.
- **Option C**: Contains an `-NHCO` group instead of `-NH₂`, which is not suitable.
- **Option D**: Similar to A, it lacks the necessary `-NH₂` group.
Based on the evaluations, **Option B** is the correct identification of the compound `X`.
### Final Answer
`X` can be identified as **Option B**.
To identify the white crystalline solid `X`, we will analyze the given chemical tests step by step.
### Step 1: Analyzing the first test
The first test states that `X` liberates `CO₂` with `NaHCO₃`. This reaction indicates that `X` must contain a carboxylic acid group (`-COOH`) or a sulfonic acid group (`-SO₃H`). Both of these functional groups can react with sodium bicarbonate to release carbon dioxide.
**Hint**: Look for compounds that contain either a carboxylic acid or a sulfonic acid group.
### Step 2: Analyzing the second test
...
Similar Questions
Explore conceptually related problems
A whiite crystalline salt imparts a violet colour to a Bunsen flame, and with hot concentrated H_(2)SO_(4) , forms a pungent gas. On treatment with an AgNO_(3) solution, this gas forms a white precipitate readily soluble in NH_(3) . The whiite crystalline salt may be:
A white crystalline compound (X) swells open heating and gives violet-coloured flame. Its aqueous solution gives the following reactions : (a) A white precipitate is formed, with BaCl_(2) in presence of HCl . (b) When treated with excess of NH_(4) OH , it gives white gelatinous precipitate. the white precipitate dissolves in NaOH and reappears on boiling with concentrated solution of NH_(4) Cl . ( c) It gives yellow precipitate with cobaltinitrite solution. Identify (X) and explain the reaction at steps a, b and c .
When H_(2)S gas a passed through an ammonical salt solution X, a slightly white precipitate is formed. The X can be:
An unknown inorganic compound (X) loses it water of crystallisation on heating and its aqueous solution gives the following reaction. (i) It gives a white turbidity with dilute hydrochloric acid solution. (ii) It decolourises a solution of iodine in potassium iodide. (iii) It gives a white precipitate with silver nitrate solution which turns black on standing. Identify the compound (X) and give chemical equations for the reaction at step (i) to (iii).
A compound (A) is greenish crystalline salt, which gave the following reactions. (i)Addition of BaCl_(2) solution to the solution of (A) results in the formation of white precipitate (B) which is insoluble in dilute HCl . (ii)On heating (A) , water vapours and two oxides of sulphur (C ) and (D) are liberated leaving a red brown residue (E) . (iii) (E) dissolves in warm concentrated HCl to give a yellow solution (F) . (iv)Solution (F) on treatment with thiocyanate ions gives blood red coloured compound (G) . Identify the compounds from (A) to (G) .
An organic compound A’ with molecular formula C_(7)H_(7)NO reacts with Br_(2) /aqKOH to give compound B’, which upon reaction with NaNO_(2) & HCl" at "O^(@) C gives C’. Compound C’ on heating with CH_(3)CH_(2)OH gives a hydrocarbon D’. Compound B’ on further reaction with Br_(2) water gives white precipitate of compound E’. Identify the compound A, B, C, D&E, also justify your answer by giving relevant chemical equations.
One cationic complex has to isomers A and B Each has one Co^(3+) five NH_(3) one CI^(Θ) and one SO_(4)^(2-) stoichiometically A give white precipitate with BaCI_(2) white B gives white precipitate with AgNO_(3) A can be .
One cationic complex has to isomers A and B Each has one Co^(3+) five NH_(3) one CI^(Θ) and one SO_(4)^(2-) stoichiometically A give white precipitate with BaCI_(2) white B gives white precipitate with AgNO_(3) (B) can be .
The metallic salt (XY) is soluble in water. (a) When the aqueous soluble of (XY) is treated with NaOH solution, a white precipitate (A) is formed. In excess of NaOH solution, a white precipitate (A) is formed. In excess of NaOH solution, white precipitate (A) dissolves to form a compound (B) . When this solution is boiled with soild NH_(4) Cl , a precipitate of compound (C) is formed. (b) An aqueous solution on treatment with BaCl_(2) solution gives a white precipitate (D) white is insoluble in conc HCl . ( c) The metallic salt (XY) forms a double salt (E) with potassium sulphate. Identify (XY),(A),(B),(C),(D) and (E) .
(i) A white solid mixture of two salts containing a common cations in insoluble in water. It dissolves in dilute HCl producing some gases (with effervescence) that turn an acidified dichromate solution gren. After the gases are passed through the acidified dichromate solution, the emerging gas turns baryta water milky. (ii) On treatment with dilute HNO_(3) , the white solid gives a solution which does not directly give a precipitate with a BaCl_(2) solution but gives a white precipitate when warmed with H_(2)O_(2) and then treated with a BaCl_(2) solution. (iii) The solution of the mixture in dilute HCl, when treated with NH_(4)Cl,NH_(4)OH and an Na_(2)HPO_(4) solution, gives a white precipitate. Q. The white precipitate obtained in (iii) consists of:
Recommended Questions
- A white crystalline solid X give following chemical test : (i) it li...
Text Solution
|
- A white crystalline compound (X) swells open heating and gives violet-...
Text Solution
|
- Statement I: Aniline on reaction with NaNO2HCl at 0^@C followed by cou...
Text Solution
|
- 2,4,6 - tribromo aniline is a product of
Text Solution
|
- A: An organic compound on diazotisation followed by reaction with alka...
Text Solution
|
- Acetaldehyde form a white crystalline precipitate mixing with a soluti...
Text Solution
|
- Which of the following on reaction with Br(2) water will give 2,4,6-tr...
Text Solution
|
- Acetaldehyde form a white crystalline precipitate mixing with a soluti...
Text Solution
|
- A compound liberates CO(2) with NaHCO(3) and also gives colour with ne...
Text Solution
|