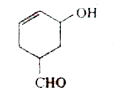
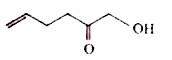
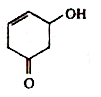
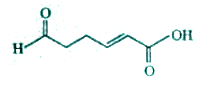
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound 'X' give following reactions X(C(6)H(8)O(2)) "GRBHPJEEORGC...
Text Solution
|
- A compound (X) with the molecular formula C(3)H(8)O can be oxidized to...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive test with NaOH ...
Text Solution
|
- Compound (A) C(8)H(6)O(2) on treatment with aq. NaOH followed by acidi...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive teast with NaOH po...
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive test with NaOH and...
Text Solution
|
- Consider the following compounds (i) C(6)H(5)COCl " " (ii) (...
Text Solution
|
- C(3)H(8)O अणुसूत्र वाले यौगिक X को C(3)H(6)O(2) अणुसूत्र वाले यौगिक Y ...
Text Solution
|
- The no. Of amines having pkb less than C(6)H(5)NH(2) among the followi...
Text Solution
|