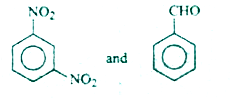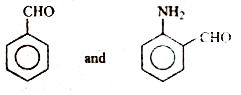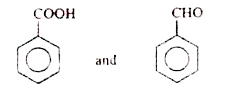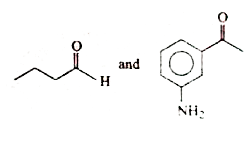A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A mixture of two organic compound gives red coloured precipitate with ...
Text Solution
|
- Which will not give precipitate with NH(4)OH Solution ?
Text Solution
|
- PhNO(2) underset(NH(4)Cl)overset(Zn)to A underset("Tollen's Reagent")o...
Text Solution
|
- A colourless mixture of two salts (A) and (B) excess is soluble in H(2...
Text Solution
|
- In analysis of third contains of mixture analysis, solid NH(4)Cl is ad...
Text Solution
|
- Laughing gas is obtained when a mixture of NH(4)Cl and …………is heated w...
Text Solution
|
- Laughing gas is obtained when a mixture of NH(4)Cl and …………is heated w...
Text Solution
|
- Which of the following pairs of cations cann be separated by adding NH...
Text Solution
|
- If a solution containing Al^(3+),Ni^(3+) and Mg^(3+) is first created ...
Text Solution
|