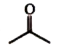
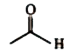
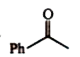
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound 'X' give positive test with 2,4 - DNP and with I(2)//NaOH c...
Text Solution
|
- Which of the following compounds will give positive iodoform test with...
Text Solution
|
- Unknown compound (A) C(5)H(10)O gives positive test with 2,4-DNP but ...
Text Solution
|
- Unknown compound (A) C(5)H(10)O gives positive test with 2,4-DNP but ...
Text Solution
|
- Compound (X)C(4)H(8)O , which reacts with 2,4-DNP derivative and gives...
Text Solution
|
- A compound gives a positive test with I(2)//NaOH and is extracted from...
Text Solution
|
- Compound 'X' C(4)H(8)O which gives 2,4-DNP derivative and negative iod...
Text Solution
|
- Compound 'X' C(4)H(8)O which gives 2,4-DNP derivative and positive iod...
Text Solution
|
- Compound (X) C(4)H(8)O gives positive haloform test but does not give ...
Text Solution
|