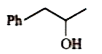
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound containing one oxygen gives red colour with cerric...
Text Solution
|
- Which can give both carbylamine test and cerric ammonium nitrate test?
Text Solution
|
- Among the following compounds which one cannot decolourise alkaline KM...
Text Solution
|
- Fe^(+3) ions in an aqueous solution gives deep red colouration with bo...
Text Solution
|
- A gas decolourises alkaline KMnO(4) solution but does not give precipi...
Text Solution
|
- The compound that decolourises alkaline KMnO(4) is :-
Text Solution
|
- The compound 'A' when trated with ceric ammonium nitrate solution give...
Text Solution
|
- Which one of the following compounds can decolourise alkanes KMnO(4) s...
Text Solution
|
- Amongst the followin,the compound which will give indoform test is
Text Solution
|