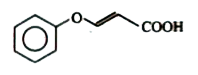
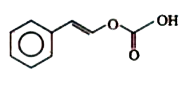
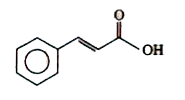
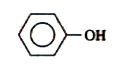
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A monocarboxylic acid decolourise Br(2)-H(2)O, on heating with soda li...
Text Solution
|
- Salicylic acid on heating with soda lime gives
Text Solution
|
- Which of the following compounds decolourise Br(2) - H(2)O and also gi...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Which of the following compounds decolourise Br(2) - H(2)O and also gi...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Which acid will not form hydrocarbon on soda lime decarboxylation ?
Text Solution
|