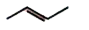A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds decolourise Br(2)-H(2)O and also give...
Text Solution
|
- Choose the correct option if compound X gives positive test with neutr...
Text Solution
|
- A monocarboxylic acid decolourise Br(2) - H(2)O, on heating with soda ...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- A monocarboxylic acid decolourise Br(2) - H(2)O, on heating with soda ...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- The complex [Cr(H(2)O)(4)Br(2)]gives the test for :
Text Solution
|