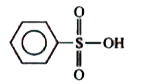
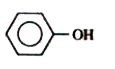
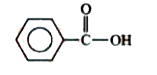
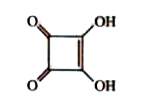
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds will not produce CO(2) on reaction wi...
Text Solution
|
- Which compound will not liberate CO(2) from aqueous NaHCO(3) ?
Text Solution
|
- Which compound give CO(2) with NaHCO(3) ?
Text Solution
|
- Which of the following will liberate CO(2) on reaction with NaHCO(3)
Text Solution
|
- Which of the following will evolve CO(2) on reaction with NaHCO(3)?
Text Solution
|
- Which of the following will liberate CO(2) on reaction with NaHCO(3) ?
Text Solution
|
- Identify number of compound from following. Which liberate CO(2) on re...
Text Solution
|
- Which compound will liberate CO(2) from NaHCO(3) ?
Text Solution
|
- Consider the following compounds, which of these will release CO(2) wi...
Text Solution
|