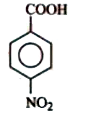
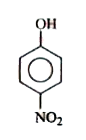
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds will react with NaNH(2) ?
Text Solution
|
- Which of the following compounds react with HNO(2)?
Text Solution
|
- Which one of the following substrate will not form benzyne when trea...
Text Solution
|
- Which of the following compound will not react with l(2)//OH^(-).
Text Solution
|
- Which of the following compound will not react with I(2)//OH^(-) .
Text Solution
|
- A compound is treated with NaNH(2) to give sodium salt. Identify the c...
Text Solution
|
- Which of the following compound will not react with I(2)//OH^(-)
Text Solution
|
- Which of the following compounds reacts with water-
Text Solution
|
- एक यौगिक NaNH(2) से अभिक्रिया कर सोडियम लवण देता है। यौगिक की पहचान कर...
Text Solution
|